The University Medical Center Schleswig-Holstein is one of the largest university hospitals in Europe with more than 80 clinics and institutes. It offers outstanding medical treatment and excellence in research and education with nearly 12,500 employees, 2,600 patient beds and more than 100,000 hospitalized patients, 240,000 outpatient cases and more than 3,000 births a year. We provide top research at the interface of medicine, science, and technology and maintain research co-operations with universities all over the world.
The Institute for Cardiogenetics was founded in January 2013.
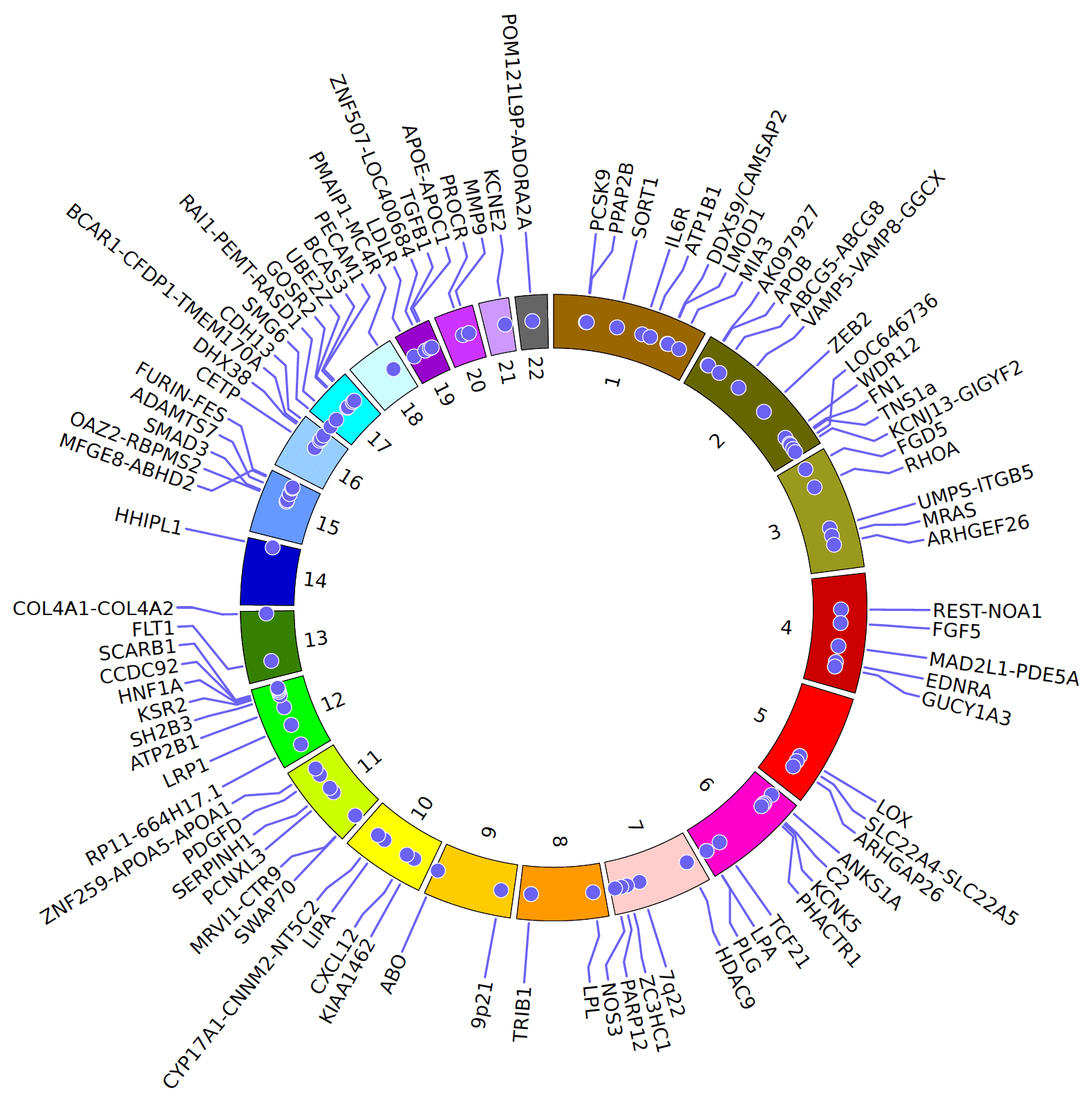
Scientists working at this institution are nationally and internationally well-recognized for their success in the field of cardiovascular genomics. Cardiovascular diseases, including coronary artery disease (CAD) and myocardial infarction (MI), are among the leading causes of mortality worldwide. Over the last five years, we identified most of the known coronary artery disease risk variants by genome-wide association studies (GWAS) and whole-exome as well as whole-genome sequencing in families (Erdmann et al. Nature Genetics 2009, Schunkert et al. Nature Genetics 2011, Erdmann et al. Nature 2013).
The primary aim of our institute is identifying further cardiovascular risk variants using GWAS as well as next-generation-sequencing and understanding the underlying pathomechanisms. We have already established several mouse and zebrafish models as well as in-vitro assays and standardized phenotyping methods in our group.
The „Institute for Cardiogenetics“ (ICG, www.cardiogenetics-luebeck.de) (Head: Prof. Dr. Jeanette Erdmann) at the University of Lübeck, Germany, is seeking for an outstanding Ph.D. student with a research interest in the field of cardiovascular genetics.
Your role:
- Establishing/maintenance of next-generation sequencing pipelines for whole-exome, whole-genome as well as panel sequencing data for large-scale population as well as family data
- Annotation of genetic variants by making use of state-of-the-art bioinformatics methods and publicly available/in-house transcriptomic/RNA-seq data.
- Presentation of scientific results at conferences, meetings, seminars
- Writing of publications under guidance of supervisors
- Contributing to supervision of master students
Your profile:
- You hold a master’s degree in bioinformatics or another relevant specialization
- You have appropriate programming skills in R, Perl or Python
- You are experienced using Linux systems including shell programming
- You have a strong background in molecular biology/genetics
- You are experienced in handling computational clusters, genetic databases such as Ensembl and/or Web Application Frameworks (Play! or Django) would be a plus
- Record of prior publications
- Personal qualities such as good teamwork attitude, multitasking ability, and communication skills (excellent in written and oral English and German language and excellent communication skills) are also essential.
What we offer:
This is a fixed-term position for 2 years, with the possibility of extension. Weekly working time is 65% of a full-time position (currently 38,50 hours per week). Working part-time is possible. Salary will be, depending on qualifications, according to the German salary scale E13 TV-L. The preferred starting date is 01.12.2017.
The UKSH has been certified as a family-friendly institution and is committed to further improve the compatibility of work and family life. The University Medical Center Schleswig-Holstein is an equal opportunity employer. People with disabilities will be given preference in case of equal qualifications.
Your Application should include:
- Letter of motivation describing previous achievements and your research interest and why you are suitable for this position (maximum 1-2 pages)
- CV including list of publications, reports, certificates
- Transcripts
- Names, e-mail addresses, and telephone numbers for at least two academic references/recommendation letters
Additional Information:
For more details on the position, please contact Prof. Dr. Jeanette Erdmann, phone: 0451 3101 8300, E-mail: jeanette.erdmann@uni-luebeck.de. Questions on administrative aspects can be addressed to Bea Roßteutscher via e-mail: karriere@uksh.de.
We are looking forward to your application. Please submit your application until o5.11.2017 indicating your earliest possible starting date as well as the reference number 20170835.219.CL.
Please apply via our online portal: www.uksh.de/Bewerbung.html?nr=20170835
More information on the University Medical Center Schleswig-Holstein can be obtained online at
www.uksh.de/karriere.
Universitätsklinikum Schleswig-Holstein
Human Resource Management | Recruiting Center