Therapy for сollagen VI-CMD
Collagen VI congenital muscular dystrophy (COL VI-CMD), previously also known as Bethlem or Ullrich myopathy, is caused by mutations in the three collagen VI genes Col6A1, Col6A2, and Col6A3.
The disease is one of more than 8,000 rare diseases with a frequency of 1-9/1,000,000. However, among the different muscle diseases, COL VI-CMD is one of the most common disorder accounting for about 30% of cases.
So far, no therapy for collagen VI-CMD is known.
Sandra Wrobel
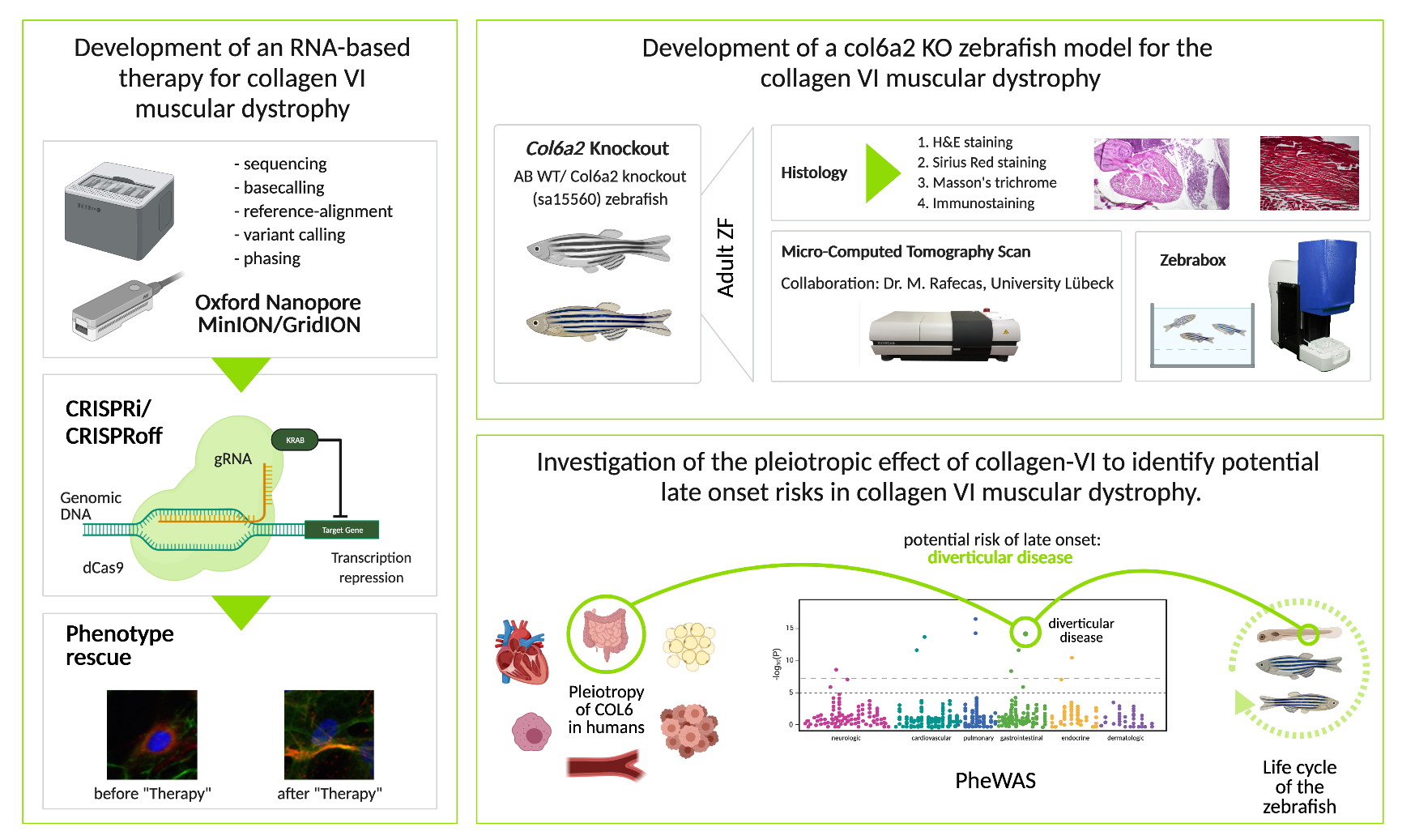
Schematic depiction of the projects at the ICG dealing with collagen VI muscular dystrophy.
Projects
RNA-based therapies attack even before the “blueprint” read from the DNA has been converted into a protein. In principle, this usually involves the suppression of mutated gene copies at the RNA level by means of so-called small interfering RNA (siRNA) or antisense oligonucleotides (ASO).
Also, in the case of collagen VI myopathy, suppression of the mutated allele should lead to therapeutic success. In the literature, first steps towards the establishment of RNA-based therapies can be found for individual mutations in the Col6A2 or Col6A3 gene. However, the first and only approach to target Col6A2 dates back to 2012 and targeted a variant leading to Ullrich CMD located in intron 9 (c.954 + 17_954 + 22del28).
We have started – together with Prof. Dr. Georg Sczakiel, director of the Institute for Molecular Medicine at the University of Lübeck – to establish an RNA-based therapy concept for the mutation p.Gly283Glu in the COL6A2 gene in the sense of individualized medicine. The goal is to generally establish this therapeutic approach targeting other dominant-negative acting glycine mutations in Col6A2 gene as well.
Furthermore, we want to develop another therapeutic approach for Col VI-CMD. Here, the aim is to use CRISPR interference (CRISPRi) to deactivate the activity of regulatory elements of Col6A2 in order to prevent transcription of the mutated allele. For CRISPRi, a Cas9 is used that no longer has a functional nuclease domain due to specific mutations (dCas9) and thus cannot cause double-strand breaks in the target sequence. Coupled with transcriptional repressors and in combination with specific guide RNAs (gRNA), dCas9 binds DNA and thus prevents transcription of the targeted gene. We will use frequent variants that are located in regulatory elements as targets to treat different Col6A2 mutations with the same gRNA. Important is that these common variants are in phase with (on the same allele as) the pathogenic variant to repress the pathogenic allele specifically. For the phasing, we enrich a 60kb region (including the gene and regulatory elements) via a method called Cas9 enrichment and sequence it using an Oxford Nanopore MinION (portable Nanopore Sequencer). The subsequent data analysis pipeline contains different command line tools.
Funded by Deutsche Gesellschaft für Muskelkranke e.V. & Cure CMD
Collagen 6 congenital muscular dystrophy (Col6-CMD) is a rare disease, which particularly affects the skeletal muscle and connective tissue. The phenotypic spectrum includes Bethlem Myopathy (mild) and Ullrich congenital muscular dystrophy (severe) that are connected by a continuum of intermediate phenotypes. The disease is caused by dominant-negative and recessive mutations in COL6A1, –A2, -A3 and in rare cases by mutations in COL12A1. Collagen VI (COL6) plays an important role in the extracellular matrix (ECM) of skeletal muscle but also in the ECMs of skin, tendon, cartilage, intervertebral discs, lenses, inner organs and blood vessels. The functions of COL6 are important in COL6-CMD, but pleiotropic functions have not yet been systematically studied.
The main aim of the study was to establish a zebrafish model to study the pleiotropic effect of a Col6-CMD variant. Furthermore, we intended to study the influence of the genetic background of TL and TU zebrafish on the severity of the disease. For this study, col6α2 knockdown (KD) is induced in zebrafish wildtype embryo using morpholino injection. The morphants are morphologically assessed by live imaging with the Acquifer imaging machine and subsequent histological examination.
As expected, col6α2 morphants (n=139) showed a broad spectrum of phenotypes, which progressed from a mild (n=45) to an intermediate (n=29) to a severe phenotype (n=65), resembling the human Col6-CMD phenotype. In addition, we observed a significant difference in mortality depending on the different genetic backgrounds. The mortality rate of the TL strain with the col6a2 KD was ~60%, whereas the mortality rate of the TU KD was ~25%.
The mutant zebrafish displayed alterations in organs including the swim bladder, gastrointestinal tract and eyes. In conclusion, we established a col6α2 zebrafish model that reflects the pathogenesis of the disease. Furthermore, initial findings indicate that the genetic background might modify the disease phenotype.
Our goal is to identify potential late-onset risks in patients with COL6-CMD by studying in-situ, in-vivo, and in-silico the pleiotropic action of collagen-VI.
Within the framework of the proposed project our specific aims are:
Aim 1.
Understanding tissue-specific phenotypes and phenotype progression associated with COL6-CMD in zebrafish larvae 5 days post fertilization (dpf), juvenile ~6 mpf and adults ≥1 ypf.
Aim 2.
Quantitative functional analysis of the larval, as well as juvenile and adult swimming behavior of col6a2 KD and KO zebrafish.
Aim 3.
Identification of potential late-onset risks for COL-6 CMD patients by leveraging PheWAS data.
Schematic depiction of the research project.
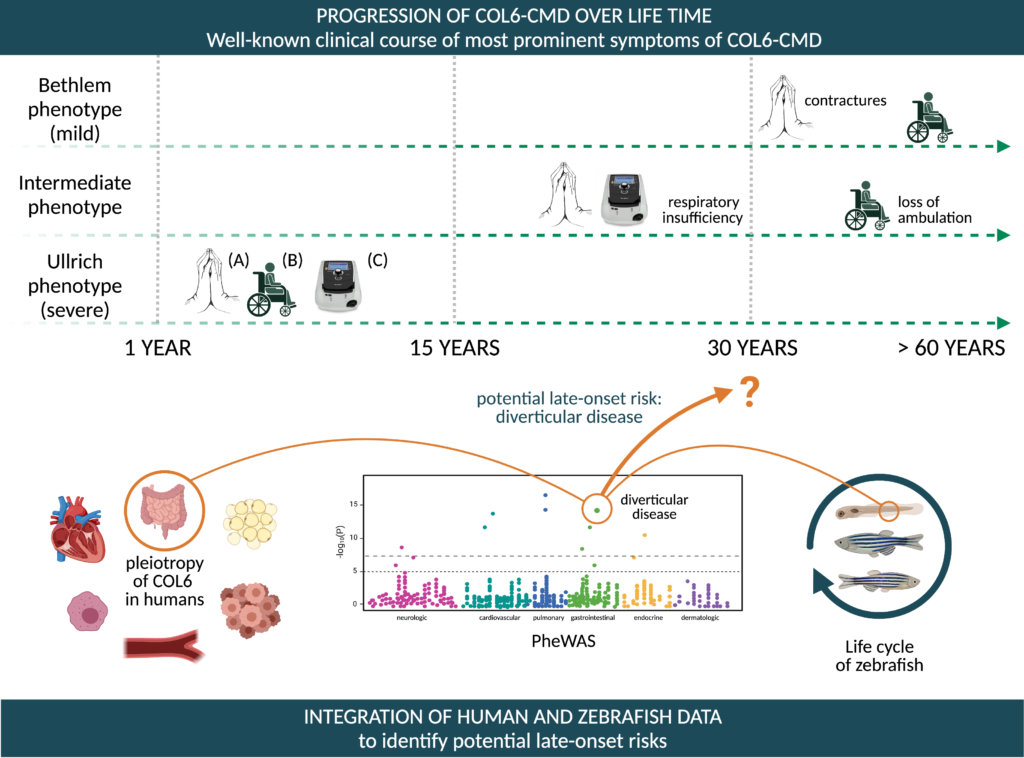
The natural history of COL6-CMD patients is mostly focused on contractures (A), muscle wasting leading to nonambulation (B), and respiratory insufficiency leading to non-invasive ventilation (C). With the proposed project we aim to integrate zebrafish and human genetic data to identify potential late-onset risks in patients with COL6-CMD. This will be achieved by studying in-situ, in-vivo, and in-silico the pleiotropic action of collagen-VI. The early recognition of potential late-onset risks in COL6-CMD patients will have a strong impact on their disease management. As an example preliminiary results from PheWAS for diverticular disease, a severe disease of the intestine, is shown. Created with BioRender.com.
Funding by Million Dollar Bike Ride 2021 Pilot Grant Program